Our Recent Articles
USP 795: NON-Sterile Compounding Ten Tangible Things You Can Do Today To Make Sure Your Pharmacy is Regulatorily Compliant
As we enter 2025, we can look back to taking stock of the year that has just rolled past us and wrapping up items both business and personal on a good note. Those of us who compound on a daily basis are well aware that the revised USP compounding chapters (USP General Chapters 795, 797, and 800) released in 2023 have been implemented and are firmly in place as the national compounding safety standards.
USP 797: Sterile Compounding Ten Tangible Things You Can Do Today to Make Your Pharmacy Compliant
Our last installment highlighted ten practical items that you could act on in short order to help you bring your non-sterile compounding program into regulatory compliance. At this time, we will attempt to achieve the same with sterile compounding in mind. This opportunity affords the average compounding pharmacist who is engaged in sterile operations an opportunity to take stock of those compounding efforts to assure that they are in alignment will all prevailing statutes, rules, and regulations. Remembering that beyond complying with all state regulations applicable, the current official USP General Chapter <797>1, is the current US standard, and as such must be taken into account.
Conduct Thorough Virtual 503B Onsite Visits
Leveraging outsourced compounders’ offerings remains a key focus in hospital pharmacy practice. According to Pharmacy Purchasing and Products’ 2024 State of Pharmacy Compounding survey, 73% of all facilities outsource at least some portion of their compounded preparations.1 When choosing a 503B outsourcing partner, performing the requisite due diligence is critical to ensuring the chosen vendor(s) operates safely and in compliance with all regulations and requirements. Developing and implementing vendor selection guidelines is essential for procuring any outsourced compounded non-sterile preparations (CNSP) or sterile preparations (CSPs) used by their organization and patients under their care.
Our Consulting Services
Clinical Presentations and Public Speaking
As a leader in CSP compounding and medication safety, LDT can develop and present on a myriad of clinical and operational topics. These areas include but are not limited to:
- Regulatory and other statutory compliance strategies, including the DQSA
- FDA activity in and around compounding pharmacies, prescribers & institutional providers (including hospitals)
- Automated Compounding Devices, Technologies & Techniques
Compounding Under - USP <795> & <797>
- Regional compounding of CSPs for your healthcare organization [503B model]
- Medication Safety & Quality Improvement topics
- Medication Quality Management & Policy Development
- Improving workflow, operational efficiencies, & policy compliance in the cleanroom
- Procurement strategies to assist in drug shortage situations
Formulary management to efficiently procure CSPs - Hazardous Drug compounding & Regulatory compliance – <USP 800> (OSHA & NIOSH topics)
- Evaluation of drug stability data & determinations of BUD of CSP formulations
- Personnel training programs for cleanroom operations
- Cleanroom design & building basics
LDT can develop and present these topics live, by webinar, or as part of your organization’s staff development efforts.

Compounding Expertise
Whether your business depends upon sterile or non-sterile pharmacy compounding or both, LDT’s pharmacists and certified technicians have a wealth of clinical and technical expertise in both cGMP and Pharmacy compounding. The delivery of compounded sterile preparations (CSPs) in the current heath care delivery model requires both technical resources and the employee training to prepare and deliver these CSPs safely. At LDT our team has over 40 years of compounding, outsourcing, and formulation experience.
We can analyze your process for regulatory and practice gaps. We can develop action plans for correction of these gaps, or analyze your operation for improvements or efficiencies to make your program better. Our experts can develop solutions that center upon the “controlled process” concept of using best-in-class” equipment and processes, in a defined and controlled way, consistently each and every time, and to document this process universally to satisfy any regulatory and oversight body.
LDT has developed compounding methodologies and accompanying documents for all types of pharmacy products including, Cardioplegia, Dialysate solutions (CVVH & CRRT), Total Parenteral Solutions (TPN), small volume preparations (SVPs) and Large volume parenterals (LVPs). Let us assist you in developing a consistent, solid library of compounding documents to reduce the possibility of errors and raise your level of quality in the mixing process.


Facility Design
LDT can assist you in the expansion or re-model of your current pharmacy space, or in a total redesign of your clean room complex. No project is too large or small; each complex is designed with your organization’s specific needs and demands in mind. All our projects use the “Pharmacist’s Eye” to work flow and efficient movement of product both in and out of the rooms. We specialize in design with careful attention to local regulation, and USP 795 & 797 considerations.
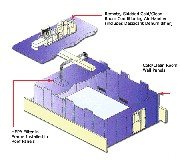
We can work with your organization’s contractors, architects, and builders to smoothly and efficiently get your complex build, validated, certified, and running. We also specialize in transition planning to assure that your critical functions are uninterrupted during any remodeling or construction. LDT has over ten years of experience in soft wall, hard wall, modular, and mobile clean room applications, please contact us for a consultation.
Compounding and GAP Analysis
The provision of contracted compounding pharmacy services from off-site providers is a growing model in healthcare. Beginning in the early 1990’s hospitals have moved high volume, fixed formulations to contract compounders (outsourcing providers) rather than compound these preparations themselves. LDT can assist at several junctures in this service paradigm.
Since November of 2013 with the enactment of the Drug Quality & Security Act (DQSA) the Congress has established Outsourcing Facilities under Section 503B of the Food Drug & Cosmetics Act. These 503B providers are expanding the areas where compounded sterile preparations (CSPs) can reach the patients who need them. Compounding these CSPs for office use, and institutional prescribing is an expanding marketplace.
With our experience in construction, developing and running successful multi-state compounding facilities under both Board of Pharmacy and cGMP regulation, LDT can guide you through the selection process if your organization, health system, or health network is contemplating such a shift in your medication delivery model.
Our services include:
- Development of your selection criteria
- Conformation of your project’s feasibility through the use of LDT’s own r-ROI™ (realistic-ROI cost modeling) for your projects budgeting.
- Formulation of your RFP
On-Site Compliance Visits (both initial and on-going) - On-going QA Visits and Audits
LDT can develop as part of your overall 503B outsourcing strategy, a comprehensive plan to accommodate the remaining compounding demands of your organization once you have moved your fixed formulations off-site. This type of strategy can reduce your organization’s overall expense in complying with Federal, State, and Local regulatory burdens, while still maintaining your organization’s ability to compound on site.
This comprehensive strategy includes:
- Clean Room Design & Contruction Expertise
- Policy & Procedure Review and Development
- On-Going QA Visits and Audits
Finally, if you are part of a larger organization or perhaps have excess capacity in your compounding suite, LDT has unique technologies and procedures to aid you in “in-sourcing” your organization’s compounding needs. By centralizing your compounding your organization may benefit from reduced overhead costs, better control, and total autonomy over your processes. This would include the review of available automation to assist you in maximizing you “in-sourcing” capacity.
LDT has several business models for “In sourcing” that may apply inside or outside your organization. Please contact us for more specific information regarding this unique opportunity.
Time & Motion Studies
LDT can assist in identifying areas for improvement and suggest creative and proven methods to maximize your staff’s capabilities.
The labor force of any organization, no matter how large or small, represents the largest investment of time, talent, and monies to any company. Inefficient use of this
single critical resource can lead to a downward budgetary spiral in any organization. LDT can assist in identifying areas for improvement and suggest creative and proven methods to maximize your staff’s capabilities.
Whether the answers lie with staff training & development, addition of technologies & software, or the launch of best demonstrated practices in key areas, LDT can develop a tailored program to fit your company’s needs and goals.
Policy & Procedures
LDT can assist in this on-going activity by analyzing your P&P collection to look for procedural gaps, regulatory inconsistencies, and to assure that your organization is operating using the latest best demonstrated practices to strive for peak efficiency.
The Policy and Procedure Manual of any company is the backbone of it quality mosaic. A proper P&P library serves as its training & teaching tools, its sales tools, as well as the company’s greatest asset in developing and maintaining the company’s quality commitment to its customers. The idea that the P&P library of any organization is a static thing is absurd and, in a health care setting, dangerous. The on-going review of your company’s standards should be a core activity that reflects your organization’s commitment to its employees as well as the community it serves.
LDT can assist in this on-going activity by analyzing your P&P collection to look for procedural gaps, regulatory inconsistencies, and to assure that your organization is operating using the latest best demonstrated practices to strive for peak efficiency.
Controlled Processes
Through the process of reviewing your organization’s activities and focusing on the possible points of “critical failure” LDT can develop systems which may minimize or eliminate these “failure points” and enhance the overall quality of your products or services.
- Compounding methodologies and documents
- Manufacturing processes and documents
- Labeling & Packaging processes
- Label standards
- TALL-man lettering & recommendations
- Bar-coding recommendations (“1-d” and “2-d” formats)
Action Planning
Once an organization has taken stock and identified key areas for improvement or gaps in current practice, the task of developing a “road-map” to get there is sometimes daunting.Once an organization has taken stock and identified key areas for improvement or gaps in current practice, the task of developing a “road-map” to get there is sometimes daunting.
Once an organization has taken stock and identified key areas for improvement or gaps in current practice, the task of developing a “road-map” to get there is sometimes daunting. Even when the course is clear, development of a solid plan while considering and balancing all the needs of the organization can also be problematic.
Let LDT bring its experience to assist you in creating a custom project plan that can be sensitive to all of these individual needs while maintaining a laser focus on the success of the project at hand. We have decades of successfully delivering time-sensitive projects, on-time and on budget.
Plans Of Correction
LDT has a broad experience in the formulation of custom POCs to address State Board, FDA form 483 deficiencies, and responses to FDA Waring Letters and other agency correspondence.
At times no matter how well an organization plans, develops, or trains it people, or how focused it is on maintaining the best and most up-to-date controlled processes, the need for an intensive corrective action is necessary. In these cases, administrative oversight and approval is needed to assure that the corrective action plan meets all the needs of the organization and perhaps satisfies the regulatory oversight in place.
LDT has a broad experience in the formulation of custom POCs to address State Board, FDA form 483 deficiencies, and responses to FDA Waring Letters and other agency correspondence. LDT maintains the largest private database of FDA public documents relating to compounding in the nation. We can mine this data to provide trending and focused reporting to assist your organization in formulating the most efficient and coherent remediation plans.
At critical times like these let LDT partner with your organization to bring it expertise in regulatory and administrative procedures to assist you in this process. We specialize in developing time-critical action plans to address these types of situations. Let LDT join your legal, administrative, and operational team to assure that your response plan is measured, appropriate, and timely.
Staff Development
Investing and developing good people is an essential piece of the “quality mosaic” which is the foundation of a solid quality system in any health care setting. Gearing your staff education and development pathways to your specific health care business need not be complicated or expensive.
At LDT we can combine audio, video, and PC based learning modules into the foundation of your “quality mosaic”. Our quality philosophy begins with competency-based learning; from there you can build a solid team. When your staff takes ownership of the quality culture that you have created, they become the most reliable teachers of new employees. This is LDT’s staff development goal.
At LDT we can access and evaluate your current system and develop a custom-tailored solution that fits your needs. Whether it is a fully custom-tailored training program or a combination of existing materials LDT can develop a solution that is correct for you and your organization.
Technology
- Automated Compounding Devices
- Implementation & Strategies
- Robotics Integration
- Telepharmacy Verification Systems
- Remote Cleanroom Environmental monitoring
- Bar coding strategies
- For Inventory accountability & tracking
- Label design & control
Testimonials
Strategic Alliance Partners









